Human Subjects (IRB)
The Institutional Review Board (IRB)
The IRB is a committee charged with carrying out the important task of reviewing minimal risk to high risk research protocols submitted by our investigators. The IRB reviews research protocols to determine and certify that the protocols conform to the regulations and policies set forth by the federal agencies – including the Department of Health and Human Services (DHHS) and the Food and Drug Administration (FDA) – regarding the health, welfare, safety, rights and privileges of human subjects. Research Integrity assists the IRB and our researchers by providing day-to-day administrative management of approximately 400 new and continuing IRB protocols.
Visit one of the options below to access our policies, answer questions, and learn how to submit a research project.
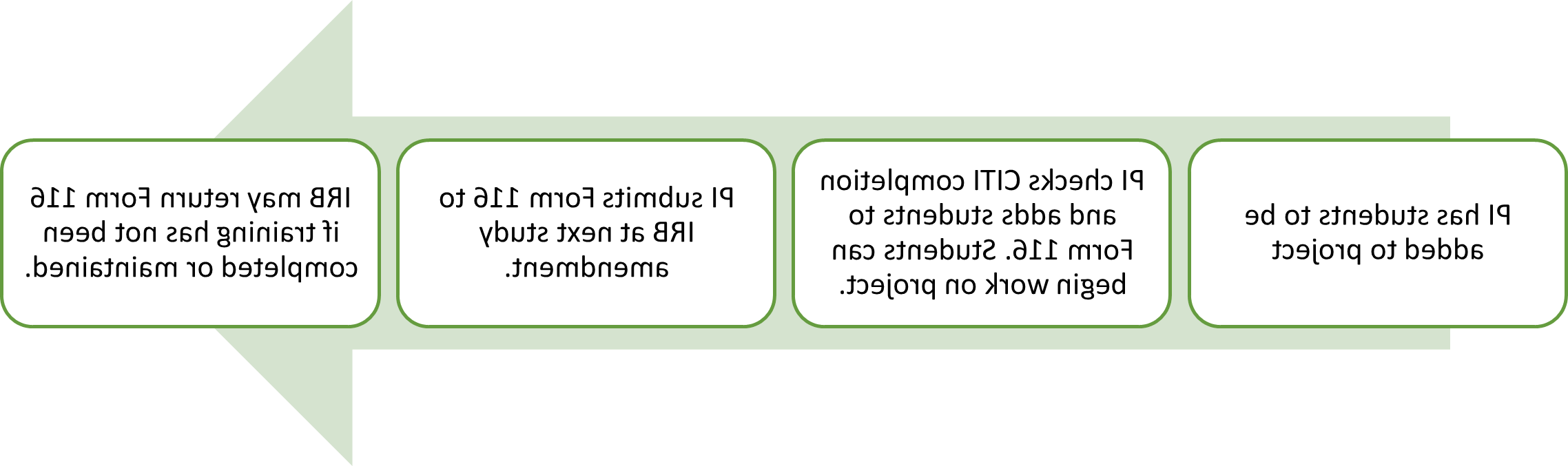
Starting October 1, 2024, minimal risk study changes in non-key study personnel do not need to be submitted via amendment. Instead, use Form 116, “Non-Key Study Personnel” to track changes and submit the form at the next study amendment. If there is a change in PI, Co-I, external collaborator, or other key personnel, these must still undergo an amendment review and approval prior to initiating the change.
We hope this new process will expedite changes to study team members. As a reminder, PIs are responsible for ensuring all study team members complete and maintain proper training prior to joining any study requiring IRB review.
IRB is:
- Providing pre-submission consultation to faculty and students for IRB proposals.
- Assisting with research protocol and informed consent preparation.
- Providing assistance in navigating IRBNet, our electronic protocol submission system.
- Coordinating monthly committee meetings and daily expedited reviews of IRB proposals.
- Developing policies, procedures, and guidance documents.
- Communicating with IRB members, Florida Atlantic faculty and students, to resolve IRB review concerns.
- Issuing IRB approvals for new and continuing studies as well as amendments and related other actions.
- Providing training on protection of human research subjects in research.
- Maintaining the university s federal assurances and providing regular updates/reports.
- Serving as liaison between external IRBs and the Division of Research.
- Interfacing with Division of Research offices to ensure coordination, consistency & compliance at all levels.
- Resolving non-compliance issues related to the protection of human subjects.
In addition to managing the IRB and IACUC functions, Research Integrity provides input and coordination on related issues involving Conflict of Interest, Good Clinical Practices, and clinical research monitoring.
Please direct questions and comments to researchintegrity@kome-shibahara.com